top of page


EU AI Act and Its Application in Developing Rare Disease Therapies for Neurological Disorders
The EU AI Act (Artificial Intelligence Act Regulation EU 2024/1689), which came into force on 1 August 2024, establishes a harmonised framework for regulating AI systems. It impacts the use of AI in medical settings, particularly when integrated with the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).

Camilla Costa
Jul 27, 20255 min read

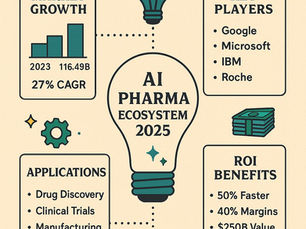
AI in Pharma 2025: Who’s Leading the Charge and Why It Matters
The convergence of artificial intelligence and pharmaceutical innovation is no longer a distant vision; it’s today’s reality. With a projected market size of $116.49 billion by the end of 2025 and a 27% compound annual growth rate (CAGR), AI in pharma is reshaping everything from drug discovery to diagnostics.

Camilla Costa
Jul 4, 20253 min read


June 2025 Regulatory Update: What MDR, IVDR & the AIA Mean for MedTech
In 2025, manufacturers of AI-powered medical devices face a new regulatory reality. The European Union has introduced the Artificial Intelligence Act (AIA). If your device falls under the Medical Device Regulation (MDR) or the In Vitro Diagnostic Regulation (IVDR), the AIA is mandatory. It’s now a core part of your compliance strategy.

Camilla Costa
Jun 11, 20258 min read


AI in Pharma: almost half a century old, news.
AI in Pharma: almost half a century old, news. AI didn’t crash into pharma. It drifted in, slowly, quietly, and no one looked twice. It’s been part of the story for nearly 50 years.

BC Regulatory Blog
Nov 1, 20242 min read
bottom of page