top of page
All Posts


The Shifting Landscape of Global Regulatory Affairs: Key Developments in Early 2026
The first few weeks of 2026 have brought significant developments from the FDA and EMA, from landmark AI collaborations to proactive measures for strengthening domestic manufacturing and securing drug supplies.

BC Regulatory Blog
Jan 253 min read


Exploring the Impact of EU AI Regulations on Biotech and SME Companies
Artificial intelligence (AI) is transforming the medical device and pharmaceutical industries at an unprecedented pace. For biotech and SME companies, leveraging AI offers exciting opportunities to innovate and improve patient outcomes. However, navigating the evolving regulatory landscape is critical to ensure compliance, safety, and market access. The European Union’s AI regulations represent a landmark framework designed to govern AI technologies responsibly. Understanding

BC Regulatory Blog
Jan 84 min read


Steps to Registering Medical Devices in Europe
Navigating the regulatory landscape for medical devices in Europe can seem daunting. However, with a clear understanding of the process and requirements, it becomes manageable and straightforward. I will guide you through the essential steps to register your medical device in Europe, ensuring compliance and smooth market entry. This knowledge is crucial for biotech and SME companies aiming to bring innovative medical products to patients efficiently and safely. Understanding

BC Regulatory Blog
Jan 64 min read


Mastering Global Regulatory Compliance: A Strategic Guide for Biotech and SME Companies
Navigating the complex world of global regulatory compliance can feel overwhelming, especially for biotech and SME companies in the medical device and pharmaceutical sectors. The landscape is constantly evolving, with diverse regulations across regions that demand precision, agility, and deep expertise. My goal is to demystify this process and provide you with clear, actionable insights to help your organisation thrive in this challenging environment. Understanding Global Reg

BC Regulatory Blog
Jan 54 min read


Navigating the Medical Device Regulatory Pathway: A Clear Guide to the Medical Device Approval Process
Bringing a medical device to market is a journey filled with regulatory checkpoints, documentation, and strategic decisions. As someone deeply involved in regulatory affairs, I understand how daunting this process can be, especially for biotech and SME companies striving to innovate while ensuring patient safety. The medical device approval process is not just a bureaucratic hurdle; it is a critical pathway that ensures every device meets stringent standards before reaching

BC Regulatory Blog
Jan 15 min read


Essential Advice for Medical Device Compliance with Medical Device Consulting Services
Understanding the Regulatory Landscape with Medical Device Consulting Services. Regulations vary by region, but the core principles remain consistent: safety, efficacy, and quality. For example, in the UK and Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) set stringent requirements. In the US, the FDA’s Quality System Regulation (QSR) governs device manufacturing and controls.

BC Regulatory Blog
Dec 23, 20254 min read


Registering Medical Devices in Europe: Key Steps for Success
Navigating the regulatory landscape for medical devices in Europe can feel daunting. However, with the right approach and clear understanding, it becomes a manageable process that opens doors to one of the world’s most significant healthcare markets. I want to share practical insights and step-by-step guidance to help you confidently move through the process of registering medical devices in Europe. This will empower your company to bring innovative products to patients faste

BC Regulatory Blog
Dec 22, 20254 min read


Achieving Market Access for Medical Devices in the UK
Entering the UK medical device market is a significant milestone for any biotech or SME company. The pathway to market access can seem complex, but with the right approach, it becomes a manageable and strategic process. I want to share insights that will help you navigate the regulatory landscape confidently, ensuring your innovative medical devices reach patients efficiently and compliantly.

BC Regulatory Blog
Dec 22, 20254 min read


Streamlining Medical Device Market Access in the UK with Medical Device Entry Consulting
Navigating the UK medical device market can be a complex journey. For biotech and SME companies, understanding the regulatory landscape is crucial to efficiently and safely bringing innovative products to patients. As someone deeply involved in this field, I know that medical device entry consulting is not just about ticking boxes; it’s about creating a strategic pathway that accelerates market access while ensuring compliance and patient safety.

BC Regulatory Blog
Dec 11, 20254 min read


Master the Keys to Entering Global Markets Successfully: A Guide to International Market Expansion
A well-structured strategy is the backbone of successful international expansion. It involves thorough market research, competitive analysis, and a clear entry plan. Here are the critical steps to consider:

BC Regulatory Blog
Dec 8, 20254 min read


The Role of Regulatory Affairs in UK Regulatory Compliance Services
Navigating the complex regulatory compliance landscape in the UK is a critical challenge for biotech and SME companies in the medical device and pharmaceutical sectors. As someone deeply involved in this field, I understand how essential it is to have a trusted partner who can guide you through the intricacies of regulations, ensuring your innovations reach patients safely and efficiently. Regulatory affairs play a pivotal role in this process, acting as the bridge between yo

BC Regulatory Blog
Dec 8, 20254 min read


Mastering FDA Compliance Consulting from the UK
Why FDA Compliance Consulting is Essential for UK Biotech and SME Companies. For biotech and SME companies, resources are often limited, and regulatory missteps can be costly. FDA compliance consulting offers tailored support that aligns with your business goals and product innovation timelines.

BC Regulatory Blog
Dec 8, 20254 min read


EU AI Act and Its Application in Developing Rare Disease Therapies for Neurological Disorders
The EU AI Act (Artificial Intelligence Act Regulation EU 2024/1689), which came into force on 1 August 2024, establishes a harmonised framework for regulating AI systems. It impacts the use of AI in medical settings, particularly when integrated with the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).

Camilla Costa
Jul 27, 20255 min read


AI in Pharma: Job Killer or Opportunity for Innovation and Growth?
In this article, we will explore how AI is reshaping the pharmaceutical landscape and examine its implications for the workforce, presenting a balanced view on the potential benefits and challenges ahead.

Camilla Costa
Jul 21, 20254 min read


Navigating the New COFEPRIS Reliance Pathway for Expedited Approvals in Mexico's Pharma Landscape
The pharmaceutical industry in Mexico is on the brink of significant change thanks to the reliance regulatory pathway introduced by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS). This new approach is designed to make the approval processes for clinical research protocols

Camilla Costa
Jul 16, 20254 min read


Navigating the Future: A Strategic Roadmap for AI-Enabled Neurotech/MedTech Device Registration and Market Access in the EU, US, and UK
Navigating the Future of Artificial Intelligence (AI) into Neurotechnology and medical technology (MedTech) is poised to revolutionise healthcare, offering unprecedented diagnostic and therapeutic capabilities.

Camilla Costa
Jul 14, 202556 min read

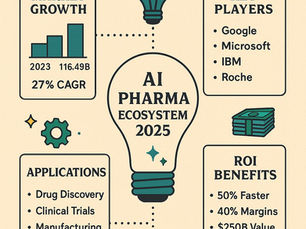
AI in Pharma 2025: Who’s Leading the Charge and Why It Matters
The convergence of artificial intelligence and pharmaceutical innovation is no longer a distant vision; it’s today’s reality. With a projected market size of $116.49 billion by the end of 2025 and a 27% compound annual growth rate (CAGR), AI in pharma is reshaping everything from drug discovery to diagnostics.

Camilla Costa
Jul 4, 20253 min read


June 2025 Regulatory Update: What MDR, IVDR & the AIA Mean for MedTech
In 2025, manufacturers of AI-powered medical devices face a new regulatory reality. The European Union has introduced the Artificial Intelligence Act (AIA). If your device falls under the Medical Device Regulation (MDR) or the In Vitro Diagnostic Regulation (IVDR), the AIA is mandatory. It’s now a core part of your compliance strategy.

Camilla Costa
Jun 11, 20258 min read


Unlocking the Potential of Machine Learning Frameworks in Medical Device Innovation and Expertise
In today's fast-paced world, technology is making remarkable changes across many sectors. Machine learning (ML) frameworks stand out as a...

Camilla Costa
May 20, 20254 min read


Structuring Clinical Trials for AI-Enabled Drug Discovery Platforms: A Comprehensive Guide
In the fast-paced world of drug discovery, artificial intelligence (AI) has become essential, transforming the way researchers identify...

Camilla Costa
May 12, 20254 min read
bottom of page